-
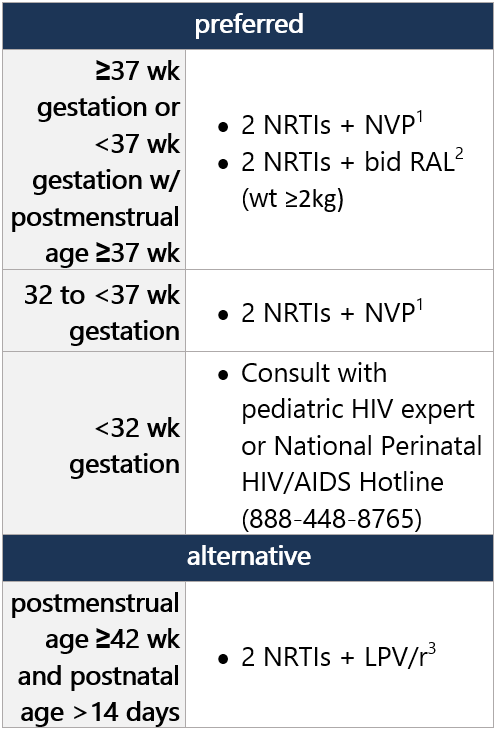
Regimen options (dual NRTI backbone + 3rd drug): Select 1 Dual NRTI backbone options: Select 1 For advantages/disadvantages of ARV components recommended for initial tx in children, see table here. For ARV regimens/components not recommended for initial tx of antiretroviral-naïve children, see table here. Footnotes 1 NVP avail. in oral solution, can be used in preterm neonates w/ gestational age ≥32 wk. NVP is not a preferred ARV agent outside of the neonatal period, due to reduced virologic efficacy, potential for toxicity, and low barrier to resistance.
2 RAL is FDA approved for term neonates weighing ≥2 kg. INSTI-based regimens are preferred for infants and children whenever possible, due to virologic efficacy, lack of drug interactions, and favorable toxicity profile. RAL produces rapid reduction in viral load but has lower resistance barrier vs. DTG and BIC. Granule form requires multistep preparation; caregivers must be educated on proper use.
3 LPV/r shouldn’t be administered to neonates before postmenstrual age of 42 wk and postnatal age of >14 days, due to potential for accumulation of LPV, alcohol, and propylene glycol. LPV/r avail. in oral solution, though has poor palatability and bitter taste, which may cause incomplete dosing if infant spits out dose. Once-daily LPV/r dosing is approved for initial tx in adults but is not recommended in children <18 yo, due to variable PK.
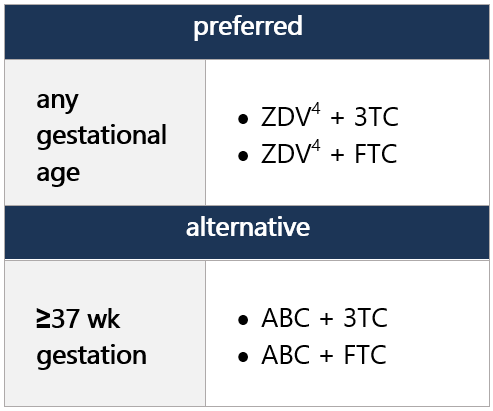
4 ZDV avail. as syrup, capsule, and tablet, as well as injectable/IV forms. Twice-daily dosing required. Hematologic toxicity may limit long-term use.
5 ABC not FDA approved for use in neonates and infants <3 mo, but WHO endorses use of ABC in full-term infants from birth, based on PK modeling. Recent data from the IMPAACT P1106 trial and 2 observational cohorts provide data on ABC safety in infants when initiated at age <3 mo. Risk of ABC hypersensitivity rxn (HSR); perform HLA-B*5701 screening before initiating ABC. Twice-daily dosing of liquid ABC recommended for initial tx; consider change to once-daily dosing for clinically stable pts w/ undetectable viral loads and stable CD4 cell counts. -
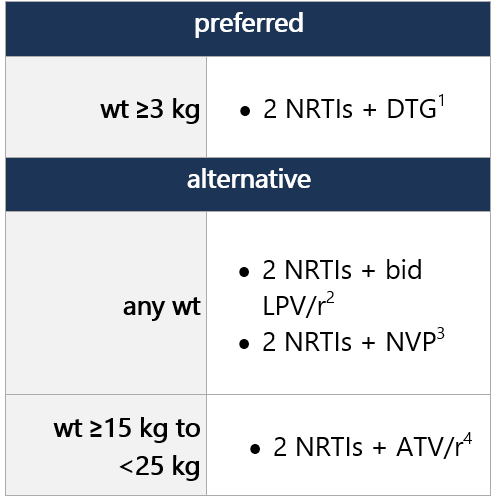
ARV regimen started during the first 29 days of life can be continued; however, the DHHS panel recommends considering switching to a DTG-containing regimen, as outlined below. DTG is preferred due to greater efficacy and durability vs. RAL and NVP, ease of DTG dispersible tablet preparation, and availability in FDC form. NRTI backbone may be continued w/ DTG, no change required. Regimen options (dual NRTI backbone + 3rd drug): Select 1 Dual NRTI backbone options: Select 1 (no alternatives suggested) For advantages/disadvantages of ARV components recommended for initial tx in children, see table here. For ARV regimens/components not recommended for initial tx of antiretroviral-naïve children, see table here. Footnotes 1 DTG is a 2nd-generation INSTI w/ a higher barrier to resistance vs. RAL. DTG dispersible tablets can be administered in infants/children ≥4 wk old and weighing ≥3 kg. Film-coated DTG tablets can be used in children weighing ≥14 kg. ABC/DTG/3TC fixed-dose combo avail. as dispersible tablets approved for children weighing ≥6 kg and <25 kg and as a film-coated tablet for children weighing ≥25 kg.
2 LPV/r shouldn’t be administered to neonates before postmenstrual age of 42 wk and postnatal age of >14 days. LPV/r avail. in oral solution, though has poor palatability and bitter taste. Poor palatability, GI side effects, and poor wt gain may limit use in this age group. Once-daily LPV/r dosing is approved for initial tx in adults but is not recommended in children <18 yo, due to variable PK.
3 NVP generally not recommended as initial tx in this age group, due to low barrier to resistance and potential for cross-resistance to other NNRTIs; may be considered if resistance or intolerance to both PIs and INSTIs.
4 ATV and RTV avail. as separate powder packets that can be mixed w/ soft food or formula and given once daily, though they have poor palatability, and tolerability may be challenging in this age group.
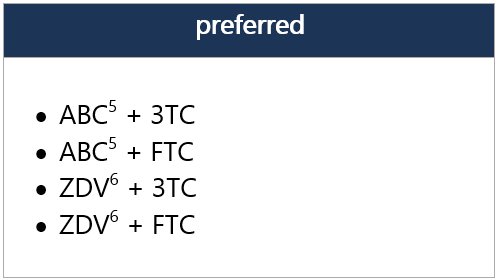
5 ABC not FDA approved for use in neonates and infants <3 mo, but WHO endorses use of ABC in full-term infants from birth, based on PK modeling. Recent data from the IMPAACT P1106 trial and 2 observational cohorts provide data on ABC safety in infants when initiated at age <3 mo. Risk of ABC hypersensitivity rxn (HSR); perform HLA-B*5701 screening before initiating ABC. Twice-daily dosing of liquid ABC recommended for initial tx; consider change to once-daily dosing for clinically stable pts w/ undetectable viral loads and stable CD4 cell counts. In pts weighing ≥6 kg to <25 kg, ABC/DTG/3TC is avail. in FDC dispersible tablets, given once daily.
6 ZDV avail. as syrup, capsule, and tablet, as well as injectable/IV forms. Twice-daily dosing required. Hematologic toxicity may limit long-term use. -
Regimen options (dual NRTI backbone + 3rd drug): Select 1 Dual NRTI backbone options: Select 1 (no alternatives suggested) For advantages/disadvantages of ARV components recommended for initial tx in children, see table here. For ARV regimens/components not recommended for initial tx of antiretroviral-naïve children, see table here. Footnotes 1 DTG is a 2nd-generation INSTI w/ a higher barrier to resistance vs. RAL. DTG dispersible tablets can be administered in infants/children ≥4 wk old and weighing ≥3 kg. Film-coated DTG tablets can be used in children weighing ≥14 kg. ABC/DTG/3TC fixed-dose combo avail. as dispersible tablets approved for children weighing ≥6 kg and <25 kg and as a film-coated tablet for children weighing ≥25 kg.
2 BIC avail. only as part of fixed-dose combo tab (BIC/FTC/TAF; Biktarvy), approved for use in children/adolescents ≥14 kg. Two strengths of the fixed-dose combo are avail., w/ dosing according to wt. Biktarvy approved for ARV tx-naïve pts; can also be used to replace current ARV regimen in pts virologically suppressed (HIV RNA <50 copies/mL) on stable ARV regimen, w/ no hx of tx failure and no known substitutions assoc w/ resistance to fixed-dose combo components. For pts unable to swallow the whole tab, tabs may be split and each part taken separately, as long as all parts ingested w/in 10min.
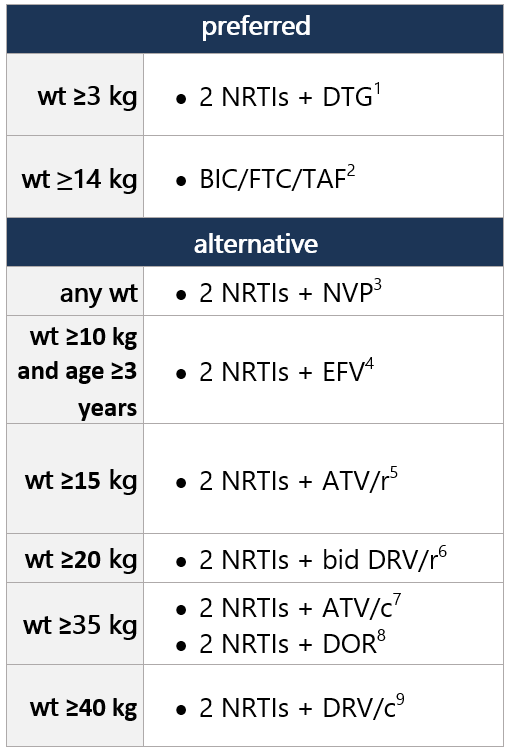
3 NNRTI-based regimens may be considered if resistance or intolerance to both PIs and INSTIs. NVP is avail. as an oral solution and tablets, for use in pts of any wt/age in this group. NVP extended-release tablets only recommended for use in children ≥6 yo.
4 NNRTI-based regimens may be considered if resistance or intolerance to both PIs and INSTIs. EFV not recommended for use in pts <3 yo, due to highly variable PK in young children, difficulty in determining appropriate dose, and side effects. EFV capsules can be opened and used as a sprinkle form for pts unable to swallow pills.
5 ATV and RTV avail. as separate powder packets that can be mixed w/ soft food or formula and given once daily, though they have poor palatability, and tolerability may be challenging in this age group. For pts who can swallow pills and weigh ≥15 kg, ATV is avail. in capsule form and can be given once daily w/ RTV tablets. Unboosted ATV not recommended.
6 DRV/r is approved for use in children ages ≥3 yo; twice-daily dosing is recommended for pts <12 yo or if the following DRV-associated substitutions are present in HIV protease: V11I, V32I, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V, and L89V. DRV is avail. as an oral solution or tablet, which can be given w/ RTV powder or tablets.
7 ATV/c is only an option in children weighing ≥35 kg and who can swallow pills. Avail. as FDC, administered once daily. Unboosted ATV not recommended.
8 DOR is avail. as a single agent and as an FDC tablet containing DOR/3TC/TDF. It’s approved for use in children/adolescents weighing ≥35 kg.
9 DRV/c is FDA approved for use in children ≥40 kg and is avail. in an FDC of DRV/c and DRV/c/FTC/TAF, both given once daily. Twice-daily dosing is not recommended w/ DRV/c. If DRV-associated resistance mutations are present, twice-daily DRV/r is recommended.
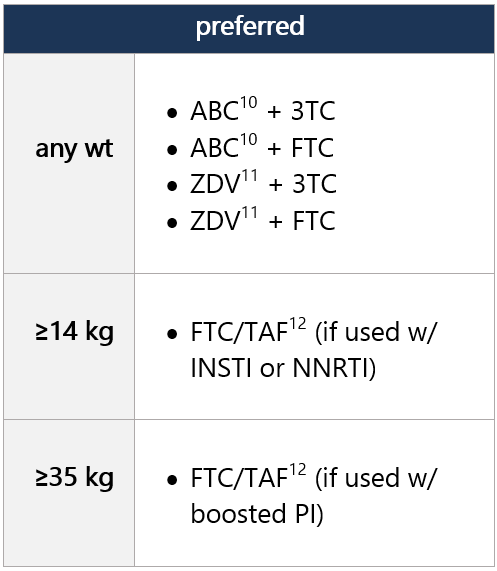
10 Risk of ABC hypersensitivity rxn (HSR); perform HLA-B*5701 screening before initiating ABC. Twice-daily dosing of liquid ABC recommended for initial tx; consider change to once-daily dosing for clinically stable pts w/ undetectable viral loads and stable CD4 cell counts. In older children who can take tablet forms (incl. FDC), initiation w/ once-daily dosing is appropriate.
11 ZDV avail. as syrup, capsule, and tablet, as well as injectable/IV forms. Twice-daily dosing required. Hematologic toxicity may limit long-term use.
12 TAF is an oral prodrug of tenofovir, w/ lower risk of renal and bone adverse effects compared w/ TDF. TAF is avail. in an FDC tablet w/ emtricitabine in 2 strengths, w/ dosing according to wt. Approved for use in children/adolescents weighing ≥14 kg w/ CrCl ≥30 mL/min. Coadministration w/ boosted ATV, DRV, or LPV increases TAF concentrations. Because no data exist on use of this combo in children <35 kg, use of FTC/TAF w/ a boosted PI in these pts is not recommended. Wt gain and incr. obesity risk observed in adults treated w/ TAF, but not clearly demonstrated in children; also assoc w/ dyslipidemia. -
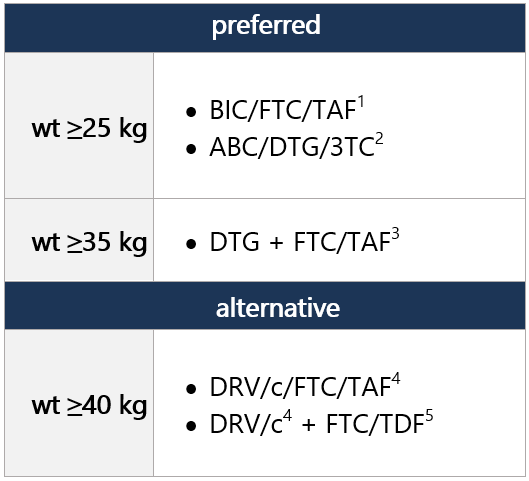
Regimen options (dual NRTI backbone + 3rd drug): Select 1 For advantages/disadvantages of ARV components recommended for initial tx in children, see table here. For ARV regimens/components not recommended for initial tx of antiretroviral-naïve children, see table here. Footnotes 1 BIC avail. only as part of fixed-dose combo tab (BIC/FTC/TAF; Biktarvy), approved for use in children/adolescents ≥14 kg. Two strengths of the fixed-dose combo are avail., w/ dosing according to wt. Biktarvy approved for ARV tx-naïve pts; can also be used to replace current ARV regimen in pts virologically suppressed (HIV RNA <50 copies/mL) on stable ARV regimen, w/ no hx of tx failure and no known substitutions assoc w/ resistance to fixed-dose combo components. For pts unable to swallow the whole tab, tabs may be split and each part taken separately, as long as all parts ingested w/in 10min.
2 ABC/DTG/3TC fixed-dose combo avail. as dispersible tablets approved for children weighing ≥6 kg and <25 kg and as a film-coated tablet for children weighing ≥25 kg.
3 TAF is an oral prodrug of tenofovir, w/ lower risk of renal and bone adverse effects compared w/ TDF. TAF is avail. in an FDC tablet w/ emtricitabine in 2 strengths, w/ dosing according to wt. Approved for use in children/adolescents weighing ≥14 kg w/ CrCl ≥30 mL/min. Coadministration w/ boosted ATV, DRV, or LPV increases TAF concentrations. Because no data exist on use of this combo in children <35 kg, use of FTC/TAF w/ a boosted PI in these pts is not recommended. Wt gain and incr. obesity risk observed in adults treated w/ TAF, but not clearly demonstrated in children; also assoc w/ dyslipidemia.
4 DRV/c-based regimens may be used if there are concerns about INSTI resistance (e.g., pts w/ hx of CAB-LA use for PrEP). DRV/c is FDA approved for use in children ≥40 kg and is avail. as part of an FDC tablet containing DRV/c/FTC/TAF (Symtuza). Twice-daily dosing is not recommended w/ DRV/c. If DRV-associated resistance mutations are present, twice-daily DRV/r is recommended.
5 TDF is avail. in powder and tablet forms, and in an FDC as FTC/TDF. Decreased BMD has been observed in adults and children, though clinical significance is unknown and routine DEXA monitoring is not recommended in children. Renal toxicity has also been observed; SCr, urine protein, and glucose should be monitored during tx.
|